How To Decrease Surface Tension Of Water
Controlling the surface tension of h2o has been a historical pursuit. Existing surfactants tin can lower it either as a monomolecular layer on water surface (Langmuir monolayers) or by forming microemulsions. In the quondam, the bulk h2o limerick is unchanged but the surface tension can be reduced from 72 mN/1000 to only well-nigh xx mN/chiliad. The microemulsion can make the interfacial tension get to one µN/m but changes the water composition. We accept shown, through measurement of capillary wave amplitudes using lengthened scattering of X-rays, that a bi-molecular layer of a iii-tailed amphiphile, preformed ferric stearate (FeSt), on water surface [1], lowers the surface tension to near 1 mN/m.
Diffuse Ten-ray scattering experiments, using an eight.04 keV X-ray axle at grazing incidence, i.e. below the disquisitional bending for total external reflection from h2o, were carried out at beamline ID10B. Preformed FeSt in chloroform solution was spread on water in a Langmuir trough, nether He atmosphere, with the diffractometer centre lying on the water surface in the trough. Two dissimilar scattering geometries were used, one employing a 'bespeak' NaI detector collected the scattered intensity in a vertical plane while the other, using a vertically mounted position-sensitive detector (PSD), collected the vertical scattered intensity contour at dissimilar positions on the horizontal plane. Intensity profile along the PSD equally well as that nerveless by the 'point' detector is proportional to the out-of-plane construction factor of the film and intensity integrated over the PSD profile is proportional to the surface fluctuation spectrum integrated forth propagation management.
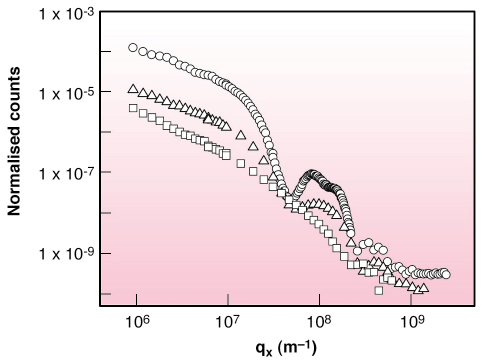 |
| Fig. 56: X-ray scattering in the vertical airplane from water (open squares), stearic acid film at |
Figure 56 shows the vertically-scattered intensity profiles from h2o, stearic acid monolayer on ferric chloride solution and movie of preformed ferric stearate on water. Whereas the acid monolayer behaves exactly every bit other Langmuir monolayers [two], the preformed fatty-acrid table salt film shows a carve up peak. The average structure gene of FeSt motion-picture show in the total scattering function yields electron density profile along film depth (Figure 57). It corresponds to the bi-molecular layer shown in schematic, composed of molecules in symmetric configuration, on meridian of molecules in asymmetric configuration with ferric ions in contact with water. The most interesting feature, shown in Figure 56, likewise as in the integrated in-aeroplane scattered profile, is the enhancement past about two orders of magnitude, of the diffuse scattered intensity of the FeSt flick at high surface pressure, i.e. loftier coverage. We ascribe this difference to enhanced conformal capillary wave height fluctuations at air-moving-picture show and film-water interfaces. The simply other possible source of large off-specular scattering is scattering from bimolecular domains, which was estimated from AFM measurements on FeSt films transferred onto Si substrates at depression moving-picture show coverage and was found to be much lower than the observed scattering. We fitted our data using the correlation role for a liquid surface, which gave excellent results over near iv orders of magnitude with surface tension = 1.iii mN/thou.
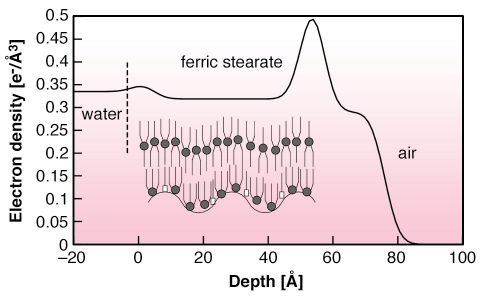 |
| Fig. 57: Electron density of compressed ferric stearate picture show on water, extracted from the X-ray scattering profile shown in Figure 56. Inset: schematics of bi-molecular film with (COO)3Fe (circle) and COOH (foursquare) groups. |
In conclusion, we take demonstrated that a bi-molecular layer of a three-tailed amphiphile drastically enhances capillary wave fluctuations on water surface due to a reduction in surface tension to ane mN/m. Different the usual Langmuir monolayers, this bi-molecular layer does not rupture under compression, but becomes thicker. This behaviour mimics folding of a membrane on a liquid surface and is closely related to the cohesive interaction brought by ferric ions.
This work was carried out under Projection 2504-ii of the Indo-French Centre for Promotion of Advanced Enquiry (IFCPAR). We would similar to thank IFCPAR for financial help.
References
[i] A. Datta, 1000. Chiliad. Sanyal, A. Dhanabalan, and S. South. Major, J. Phys. Chem. B 101, 9780 (1997).
[2] C. Fradin, J. Daillant, A. Braslau, D. Luzet, M. Alba, and M. Goldmann, Eur. Phys. J. B i, 57 (1998)
Principal Publication and Authors
A. Datta (a), S. Kundu (a), M. K. Sanyal (a), J. Daillant (b), D. Luzet (b), C. Blot (b) and B. Struth (c), Phys. Rev. E, 71, 041604-1 – 041604-vii (2005).
(a) Saha Institute of Nuclear Physics, Kolkata (India)
(b) LIONS, CEA, Saclay (France)
(c) ESRF, Grenoble (France)
How To Decrease Surface Tension Of Water,
Source: https://www.esrf.fr/UsersAndScience/Publications/Highlights/2005/SCM/SCM1
Posted by: mccuskermente1947.blogspot.com


0 Response to "How To Decrease Surface Tension Of Water"
Post a Comment